Gene Therapy
Webinar Recording: Immunogenicity assessment of gene therapy compounds: discussion on current and future concepts
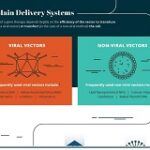
After a series of setbacks in the nineties, several gene therapies have now been approved by health agencies worldwide. As a consequence, the number of these…
Immune responses to AAV vectors: overcoming barriers to successful gene therapy
Mingozzi F, High KA. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood. 2013;122:23-36. Abstract Gene therapy products for the treatment of…