Discovering New Drugs: The Importance of the Discovery Phase in Drug Development
The discovery phase of drug development is an early, critical part of the process where potential drug targets are identified and validated to select the most promising candidate for advancement.
BioAgilytix is a key partner in drug discovery, providing unrivaled expertise and technologies in bioanalysis for our partners. Our proven capabilities in assay development, biomarker analysis, and pharmacokinetic assessments allow sponsors to efficiently identify, validate, and optimize lead candidates for successful drug development programs.
Stages in the drug development process
The drug development process is a complex and rigorous journey that encompasses several stages, starting from the initial discovery of a potential drug candidate and progressing through preclinical testing, clinical trials, regulatory approval, and post-marketing activities. This process aims to ensure the safety, efficacy, and quality of pharmaceutical products before they reach patients.
BioAgilytix is the partner trusted most by leading biopharma organizations to usher candidates through all stages in the drug development process.
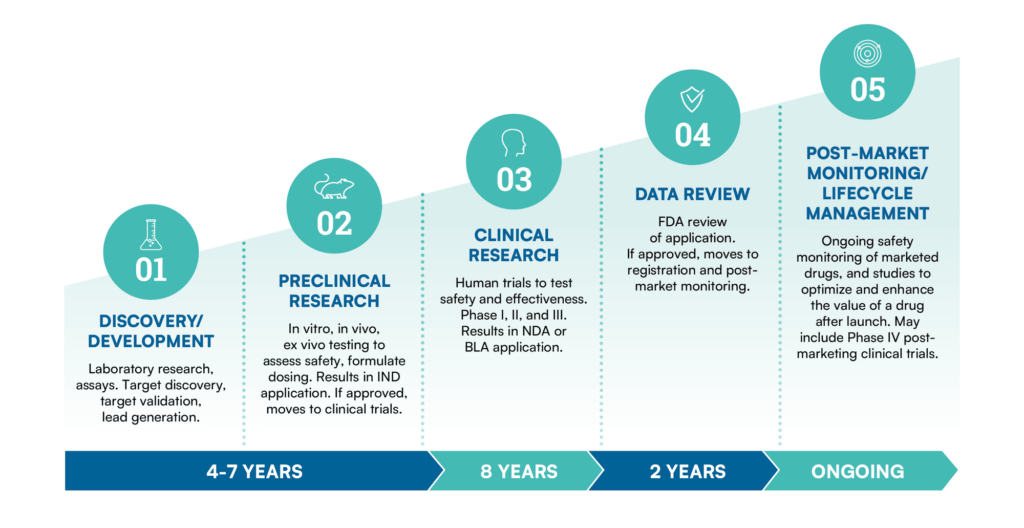
Learn more about how BioAgilytix is helping leading companies achieve success in each step of the drug development process:
Steps in the drug discovery process
There are four key stages of the drug discovery process which include target identification, target validation, lead compound identification, and lead optimization. BioAgilytix is uniquely positioned to advance our sponsors’ therapeutic candidates through each of these stages in drug discovery.
Target identification is the first step in the drug discovery process, where researchers identify specific molecular targets within the body that play key roles in a disease or condition. By understanding the underlying biological mechanisms and pathways associated with the disease, scientists can identify potential targets that can be modulated to achieve therapeutic effects. Target identification involves a combination of experimental and computational approaches, including genomic, proteomic, and bioinformatic analyses.
Target identification is essential to the discovery process. Once a therapeutic target is identified, the subsequent steps in drug development—such as compound screening and lead optimization—can be clearly defined, ultimately leading to the development of effective therapeutic strategies.
After target identification, researchers conduct extensive experiments and studies to validate whether modulating the target will result in the desired therapeutic effects. This is called target validation.
Target validation establishes the relevance and potential efficacy of altering the target, guiding the approach to identifying a new drug candidate with therapeutic benefit and reducing the risk of costly failures in later stages of a drug program.
Lead compound identification is a pivotal next step in the drug discovery process where researchers identify and select promising compounds that act on a validated target. Typically utilizing high-throughput screening methods, discovery scientists will evaluate a large library of potential active chemical compounds to identify those that interact with the target and produce the desired pharmacological effects.
Lead compound identification includes rigorous analysis of compound potency, selectivity, pharmacokinetics, and safety profiles. Successful identification of lead compounds provides a starting point for further optimization and development, ultimately leading to potential therapeutic treatments.
After a lead compound (or a set of compounds) is identified, it will be refined and optimized to enhance its potency, selectivity, and pharmacokinetic properties. Additional changes may be necessary to improve the expected safety profile by minimizing potential toxicity. Through cycles of chemical modifications, structure-activity relationship studies, and biological evaluations, researchers work to improve the therapeutic potential of the lead compound.
Lead optimization requires a careful balance between optimal potency and expected safety to develop a compound that maximizes the therapeutic benefit while maintaining a safe overall profile, thus paving the way for successful preclinical and clinical testing at later stages of drug development.
BioAgilytix’s expertise in the drug discovery phase
BioAgilytix contributes to the discovery phase of drug development by leveraging our extensive expertise and advanced technologies to design tailored bioanalytical strategies for discovery programs. Our capabilities in custom assay development, including designing novel cell-based assays, enable efficient analysis and characterization of potential drug candidates.
With our deep scientific expertise and state-of-the-art platforms, BioAgilytix plays a crucial role in supporting pharmaceutical and biotechnology companies in the rigorous evaluation and validation of drug targets and compounds during the discovery phase. We have a proven history of getting it right from the start—no matter how challenging the program may be.
Frequently asked questions about drug discovery
Bringing a new drug to market typically takes around 10 to 15 years, but timelines can vary dramatically. Factors such as drug modality, disease target, trial success rate, and specific regulatory requirements influence the duration of the drug development process.
Common early drug development issues include safety concerns, efficacy challenges, formulation and delivery complexities, understanding pharmacokinetics and pharmacodynamics, regulatory compliance, intellectual property protection, and securing sufficient funding and resources. Addressing these issues requires careful planning, collaboration, and a systematic approach to maximize success.
Speak to a scientist today
Learn more about how BioAgilytix can help you reach your discovery drug development goals