Clinical Drug Development Process: Stages, Phases, and Timeline

The clinical stage is a critical part of the drug development process, where potential therapies are tested on human subjects to evaluate safety, efficacy, and dosage. Companies developing therapeutics face distinct challenges during the clinical stage of development, including:
- Safety and efficacy concerns
- Regulatory requirements
- Immunogenicity issues
- Growing competitive landscape
BioAgilytix is the leading CRO partner to help sponsors overcome these challenges. We are the partner trusted most by leading biotechnology and pharmaceutical organizations for our unrivaled program experience and deep scientific expertise in both large and small molecule clinical development. We have the depth and breadth of experience to optimize resource utilization, ensuring successful delivery of data for clinical trials.
What is the clinical phase in drug development?
The clinical stage of drug development involves testing potential therapies on human subjects to evaluate their safety, efficacy, and dosage. It consists of three distinct phases: Phase 1, 2, and 3
Phase 1 clinical trials
Phase 1 of the clinical trial process involves testing a potential therapy on a small group of healthy volunteers. The primary objectives are to evaluate the drug’s safety, determine the appropriate dosage, and identify any potential side effects. This phase also helps in understanding the drug’s pharmacokinetics—how it is absorbed, distributed, metabolized, and excreted by the body. The main goal of Phase 1 is to establish a safe starting dose that can be used in subsequent trials.
Phase 2 clinical trials
Phase 2 expands the study population to include patients who have a targeted disease or condition. This phase aims to assess the effectiveness of the drug in treating the specific condition and to further evaluate its safety and dosage regimens. It provides valuable preliminary data on the potential benefits and side effects of the drug in a more targeted patient population.
Phase 3 clinical trials
Phase 3 trials are typically conducted on a larger scale, involving hundreds or even thousands of patients. The primary objective is to confirm and expand on the effectiveness and safety profile of the drug. Phase 3 trials typically involve comparing the drug against existing standard treatments or a placebo. These studies collect comprehensive data on the drug’s efficacy, optimal dosage, long-term effects, and safety across a broader patient population. The data generated from Phase 3 trials is crucial for regulatory submissions and potential approval of the drug for market distribution.
Learn More about BioAgilytix’s Australian Advantage

How does BioAgilytix contribute to the clinical drug development process?
During clinical drug development, each phase builds upon the knowledge gained in the previous phase. With our depth of experience and scientific focus, BioAgilytix is uniquely positioned to help our partners succeed in gathering robust data supporting regulatory approval and commercialization. We have a deep history of successful assay design, , data generation, and compliance with ethical and regulatory guidelines. We help drug sponsors stay on target for budget and timeline.
Stages in the drug development process
The drug development process is a complex and rigorous journey that encompasses several stages, starting from the initial discovery of a potential drug candidate and progressing through preclinical testing, clinical trials, regulatory approval, and post-marketing activities. This process aims to ensure the safety, efficacy, and quality of pharmaceutical products before they reach patients.
BioAgilytix is the partner trusted most by leading biotechnology and pharmaceutical organizations to usher candidates through all stages in the drug development process.
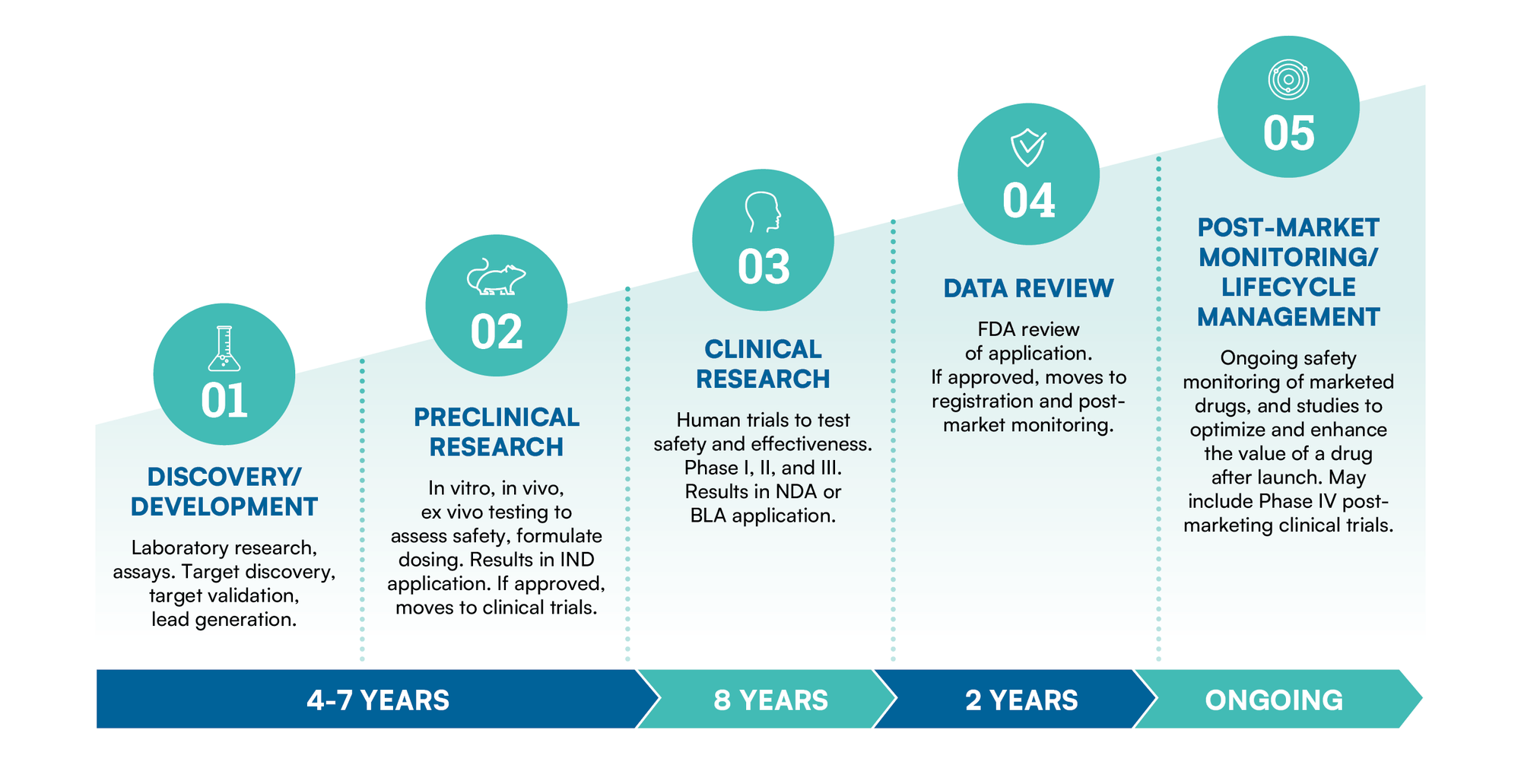
Learn more about how BioAgilytix is helping leading companies achieve success in other stages of the drug development process:

Gaining regulatory approval for drugs
The regulatory review process involves close collaboration between a drug sponsor and regulatory authorities to ensure that a drug meets standards for safety, efficacy, and quality before approval is granted.
BioAgilytix provided bioanalytical support for nearly a third of all therapeutics that received BLA approvals from the FDA between 2018 and 2022. Contact us today to learn more about how BioAgilytix scientists can help your drug candidate succeed.
People also ask:
The stages of the drug development process include Discovery, Preclinical Testing, Clinical Trials (Phase 1, 2, 3), New Drug Application (NDA) or Biologics License Application (BLA) Submission, Regulatory Review, Approval, Post-Market Approval Surveillance, and Lifecycle Management.
The goals of clinical drug development are to evaluate the safety and efficacy of a potential therapy, determine the appropriate dosage and administration regimens, assess its benefits and risks in specific patient populations, and gather comprehensive data to support regulatory approval for market distribution.
Phase 4 clinical trials, also known as post-marketing studies, occur after a drug is approved and on the market. They aim to gather additional information on the drug’s safety, long-term effectiveness, and potential benefits or risks in larger patient populations, providing real-world evidence for further evaluation and monitoring.