Experience, expertise, and scale, positioned to meet your needs wherever you operate, with a core strength in bioanalysis to successfully navigate therapeutic development from start to finish.
We take a uniquely consultative approach to
every project.
As a leading global bioanalytical laboratory, we help our partners navigate the complex scientific and regulatory challenges inherent in the bioanalytical testing of new therapies to improve patients’ lives. Our experienced scientists deliver bioanalytical projects across all phases of drug development for pharmaceutical and biotechnology organizations of all types and sizes.
Leveraging the industry’s most complete set of capabilities and expert resources, our scientists working with your scientists to proactively anticipate assay challenges, and keep you on track. Our expertise, regulatory track record, focus on quality and continuity of scientific oversight, avoiding costly delays, so your therapeutic advancements get to market sooner.
What sets us apart
Choosing the right partner is critically important. Bioanalysis is a crucial part of every stage of development of new therapies, and delays and missteps can derail an otherwise promising therapy. BioAgilytix is uniquely qualified to help you keep your study on-track. Here’s why.
Specialization in bioanalysis
Our sole focus is on innovative and reliable bioanalytical testing. Our passion for the science of assay development, biomarker analysis, and pharmacokinetic assessments drives our delivery of premium, tailored services.
Scientist-to-scientist approach
We take a uniquely consultative approach to every project, working scientist-to-scientist in continuity with your internal teams, proactively anticipating challenges, accelerating timelines through discovery, clinical development, market launch and post-market surveillance. We offer flexible solutions and responsive communication throughout the entire drug development process.
Advanced technology
Our network of global laboratories utilizes industry-leading platforms and techniques to support effective and efficient bioanalysis for new therapies.
Regulatory compliance
BioAgilytix maintains rigorous quality standards and regulatory compliance, meeting or exceeding the requirements of international regulatory agencies, such as the FDA, EMA, and others.
Worldwide presence
With laboratories in most of the leading biotech and pharmaceutical areas in the US, Europe and Asia Pacific, we provide end-to-end program success.

Scientific experience that runs deep
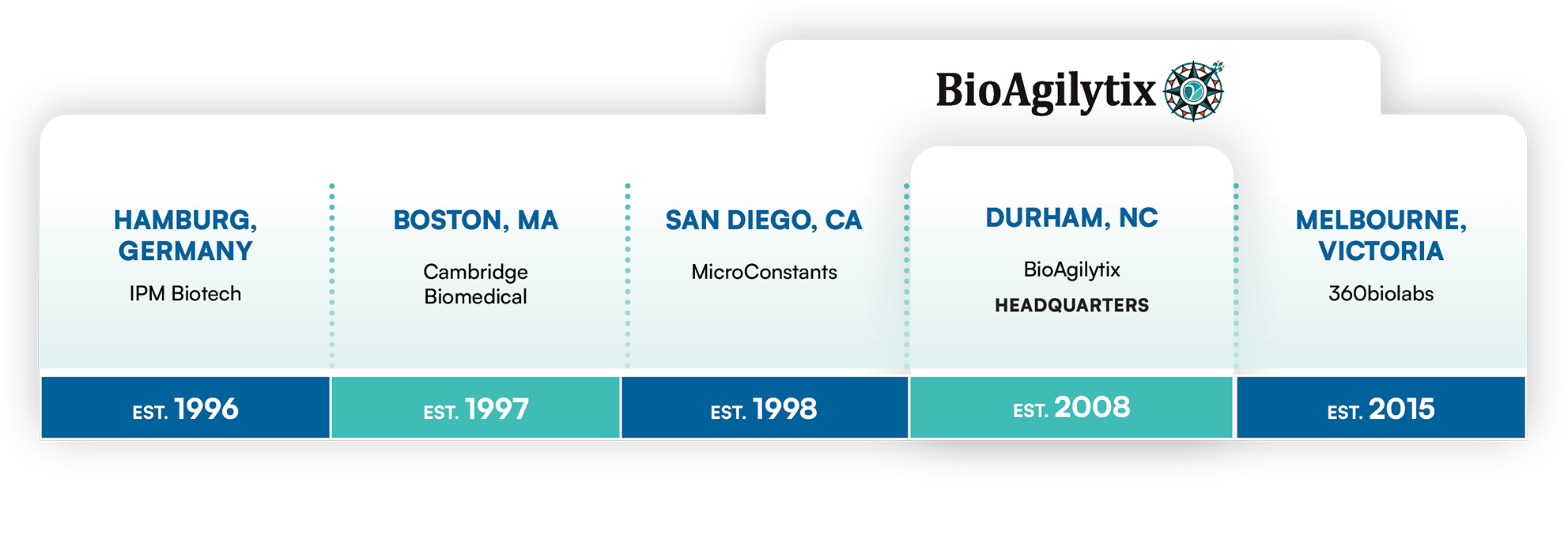
Our commitment to science, innovation, and quality is deeply rooted in our company story which began nearly thirty years ago. The BioAgilytix of today is an amalgamation of the most progressive organizations and informed minds in the bioanalytical testing field.
Three decades in the business of scientific excellence
By acquiring four leading bioanalytical laboratories based in different drug development hubs around the world, BioAgilytix provides a broad scope of global solutions.
Our People and their depth of scientific knowledge
We believe that the only way to maintain the standard of quality that our customers expect is by maintaining a “deep bench” – a scientific team that has the breadth and depth of experience required to overcome bioanalytical complexities in any phase of development.
With more than 1000 colleagues from over 50 countries, we employ a highly skilled, diverse team that is the engine for our critical work on behalf of our pharmaceutical and biotechnology partners.
Our global science team averages 10+ years of bench experience, with over 50% PhD/master’s-level scientists. Each brings their own real-world experience in the stringent scientific and quality demands of biologics development to benefit your study.

Do you have a passion to advance bioanalytical innovation?
Come join our collaborative and diverse team. We have worked diligently to build a positive culture that supports your career journey, the company’s core values, our customers’ goals, and patients’ needs.

Segments We Serve
Every life sciences segment is unique – as is every company and every study. With 30 years of broad experience, including having delivered bioanalysis services to 21 of the top-25 global pharmaceutical and biotechnology companies, BioAgilytix has learned the nuances of conducting successful, low-risk projects across multiple segments within the clinical trial space. Specifically, we support:
- Pharmaceuticals
- Biotechnology
- Biomedical technology
- Agricultural biotechnology
- Animal health
- Emerging, mid-sized, and large organizations
Commitment to Quality
Our commitment to quality leads our partners to trust that they are receiving the most accurate, precise, and comprehensive high-quality bioanalytical testing services.
Commitment to Environmental, Social, and Governance (ESG)
We recognize that our success is intertwined with the well-being of our planet, our people, and the communities we serve.
Awards
The BioAgilytix culture of integrity-driven leadership, in which everyone operates with mutual accountability and respect, is the driving force behind its recognized business excellence.

8 time
winner since 2013

11 time
winner since 2011

4 time
winner since 2014