Fast and Accurate Gyrolab Assay Services for Biopharmaceuticals
BioAgilytix uses the Gyros platform to perform immunoassays using this proprietary microfluidics system. Immunoassays on the Gyrolab generate quick, reproducible results using much smaller sample volumes than traditional ELISA techniques. Gyrolab automates robust immunoassays run with identical microfluidic channels using highly sensitive, laser-induced fluorescence (LIF) detection.
BioAgilytix’s Gyrolab platform expertise
When your immunoassay needs high precision and high throughput, but low sample volumes and tight timelines are complicating factors, BioAgilytix makes Gyrolab our go-to technology. Its flow-through affinity column format minimizes matrix interference while using nanoliter-sized sample volumes. We know how to best leverage the Gyros platform’s unique capabilities to make the most of your precious sample volumes and can quickly develop the assays we need to deliver fast, efficient, and reliable results. Collaborate with our scientific team and we’ll help you determine how the Gyros can best be used to support your program.

Ideal Gyrolab applications:
For nanoliter-scale immunoassays
The flexibility of Gyros’ technology makes it a viable platform for any phase of your drug program. In the discovery stage, development is subject to aggressive timelines, and large and diverse sample numbers must be analyzed — typically with limited sample and reagent volumes. For such projects, Gyrolab enables serial sampling on reduced volumes, with rapid time to results. In comparison, for regulated bioanalysis Gyrolab’s automation increases method robustness and shortens project timelines by yielding high-quality data for fast decision-making.
Overall, the Gyros platform is valuable when used in studies with small sample volumes or microsampling, but which require frequent collections. It’s also used for a range of complex matrices such as CSF or sputum. The BioAgilytix team uses our Gyrolab xP workstations to support our work with biomarkers, pharmacokinetics (PK), and lot release testing, but we have the expertise to tailor the platform to meet your unique assay specifications.
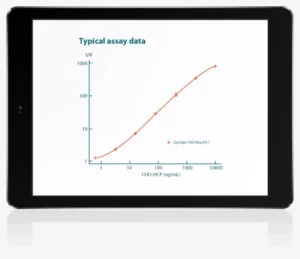
Key Gyrolab benefits:
high precision and fast total assay time
By significantly reducing sample and reagent consumption, Gyrolab removes time- and cost-consuming roadblocks faced with limited sample availability in more traditional methods. The platform’s ability to automate assay workflows eliminates many of the steps where human error can cause variability in assay performance, and yields rapid but accurate results for fast, effective decision-making. The technology behind the Gyros platform also minimizes dilutions and assay repeats by reproducibly measuring concentrations over a broad dynamic range. This simplifies the assay timelines and increases throughput.
There is little need for sample pretreatment, and assays can be run in a diverse range of matrices. This flexibility extends to assay formats as well. Gyrolab CDs provide a foundation on which to design assays to quantify proteins with a suitable antibody binding pair.

How the Gyrolab platform works
Gyrolab xP uses proprietary Gyrolab CD technology, which is engineered with highly reproducible nanoliter microfluidics for development of robust, nanoliter-scale immunoassays exhibiting broad dynamic ranges. Within each CD itself, a volume definition chamber delivers ultra-accurate sample volumes, with hydrophobic breaks to ensure consistency in volume delivery. An overflow channel eliminates the need for manual pipetting accuracy, and a 15-nL affinity capture column mitigates nonspecific binding.
Once CDs are loaded into the Gyrolab workstation (up to five discs in a series), the platform performs parallel sample processing via highly-sensitive, laser-induced fluorescence detection. The CDs are spun at controlled speeds to ensure optimal binding and uniform conditions for all assays. This is possible through precise, automated control of centrifugal and capillary forces, which directs liquid flow through nanoliter-scale microfluidic structures contained within the CD.
Webinar
Gyrolab®: Case studies in assay development & validation
Webinar